Electrodialysis
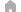
- Products
- FORBLUE SELEMION™
- Electrodialysis
Using SELEMIONTM Ion-Exchange Membranes for Electrodialysis
Solution components can be isolated, desalinated, concentrated, or extracted.
- Electrodialysis involves desalination and concentration, using DC current as the driving force.
- Unlike ion-exchange resins, ion-exchange membranes do not need to be regenerated, reducing chemical use.
- Non-ionized compounds do not pass through ion-exchange membranes. Therefore, the membranes separate organic matter from salts.
Principle of electrodialysis
Multiple pairs of alternating cation- and anion-exchange membranes are laminated with two kinds of spacers, Desalination chamber (desalination) and Concentration chamber (concentration), in between them. Each end of stack is equipped with a pair of electrodes. Supplying Desalination chamber with raw solution causes the cations to move toward the cathode (negative pole), thereby passing through the cation exchange membrane and moving to Concentration chamber, adjacent to the right. Since the cathode side of Concentration chamber, however, is partitioned with an anion-exchange membrane, the cations cannot move to Desalination chamber, further to the right. In the same way, the anions move from Desalination chamber to Concentration chamber, to the left. As a result, the solution in Desalination chamber will become desalinated, while that in Concentration chamber will become concentrated.
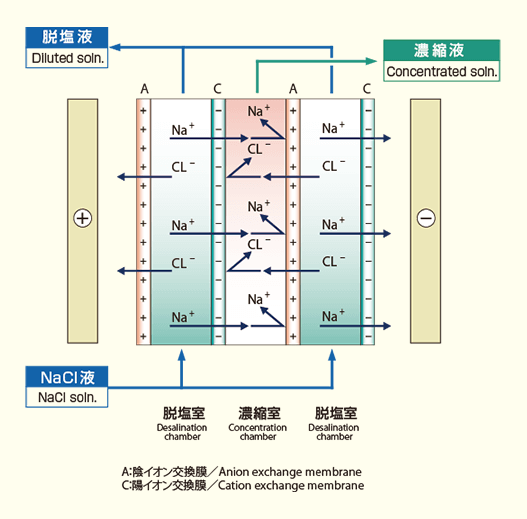
Process flow of electrodialysis
In the electrodialysis process, solution is repeatedly circulated through the electrodialyzer to desalinate/concentrate.
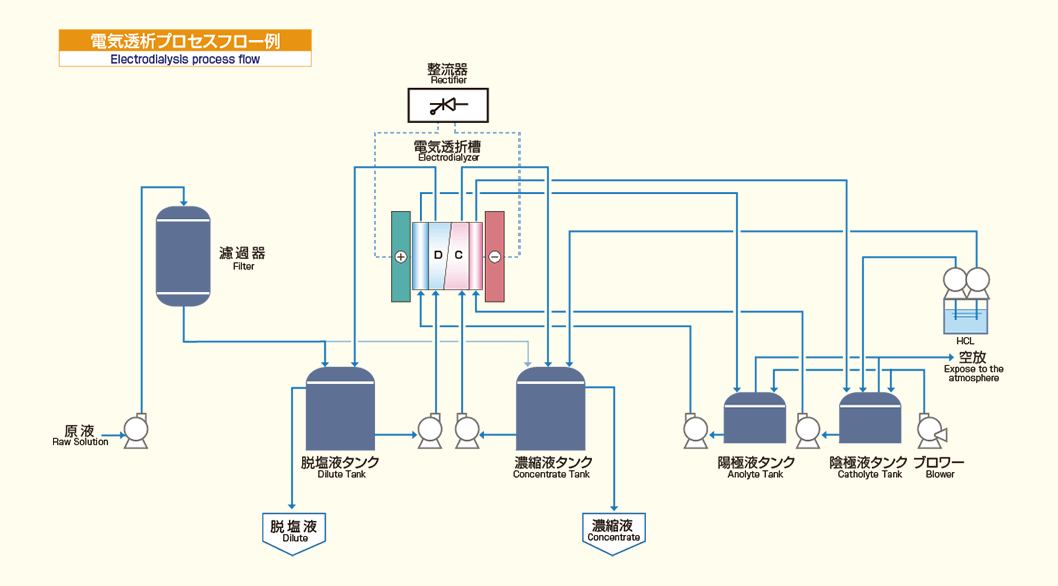
An example flowchart of an electrodialysis process
Desalination and concentration by dialysis are the most basic and widely used ion-exchange membrane processes.
Click here for an applicable areas.
Foods and drinking water
- Manufacture of table salt from seawater concentration
- Desalination of various foods
- Manufacture of drinking water by desalinating and denitrifying well water
Drainage processing and recycling
- Desalination and recycling of drainage from various processes
- Desalination and recycling of drainage from activated sludge
- Desalination of leachate from landfill site
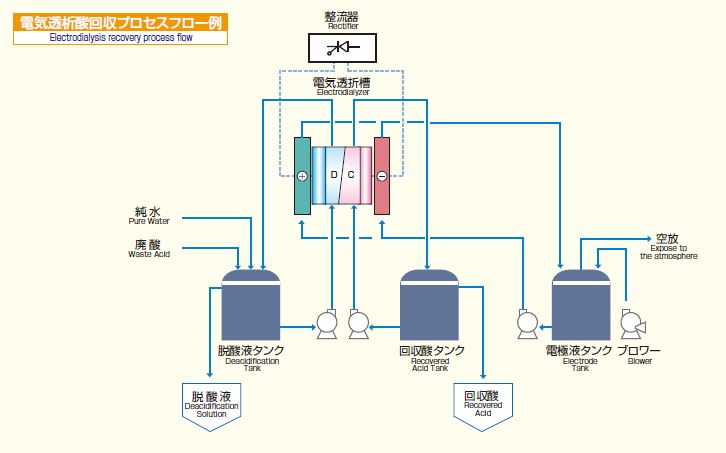
Electrodialysis acid recovery
Acid recovery by electrodialysis uses a hydrogen ion-selective membrane developed by AGC. This membrane transmits hydrogen ions selectively while resisting the transmission of metal ions, making it possible to use electrodialysis for the separation, concentration, and recovery of acids.
Flowchart of electrodialysis acid recovery
In the acid recovery process as well, raw solution is repeatedly circulated through the electrodialyzer and deacidified/concentrated.
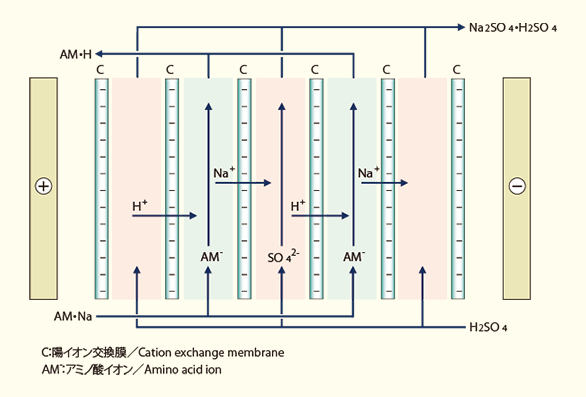
Substitutional electrodialysis
Substitutional electrodialysis using SELEMIONTM is an applied process used to manufacture amino acids, various organic acids, and base compounds. In this process, counterions are replaced with acids or alkalis, depending on the type of membrane used, to manufacture acid derivates or base derivatives from neutral salts.